Group of Biomedical Neuroengineering
| Responsible | Eduardo Fernández Jover |
RESEARCH SUMMARY
We are a multidisciplinary group of specialists in various fields such as engineering, biology, computer science, robotics, medicine, etc. Our main objective is basic and applied research in visual pathologies, optogenetics, the study of therapies against visual neuropathies, the design and development of robotic devices and systems that help improve cognitive and communicative capacity, health and physical capacity. of people with motor and sensory disabilities…
RESEARCH LINES
Line 1: Development and validation of a laser-induced choroidal neovascularization model
The laser-induced choroidal neovascularization model is an established method used to evaluate therapies for wet age-related macular degeneration (wet AMD), a leading cause of blindness in our older adult population. The main objective of this line is to obtain a validated model to experiment with therapies and treatments for the control and cure of this pathology with the aim of moving to clinical phases in humans with the treatments tested in the animal model in the future.
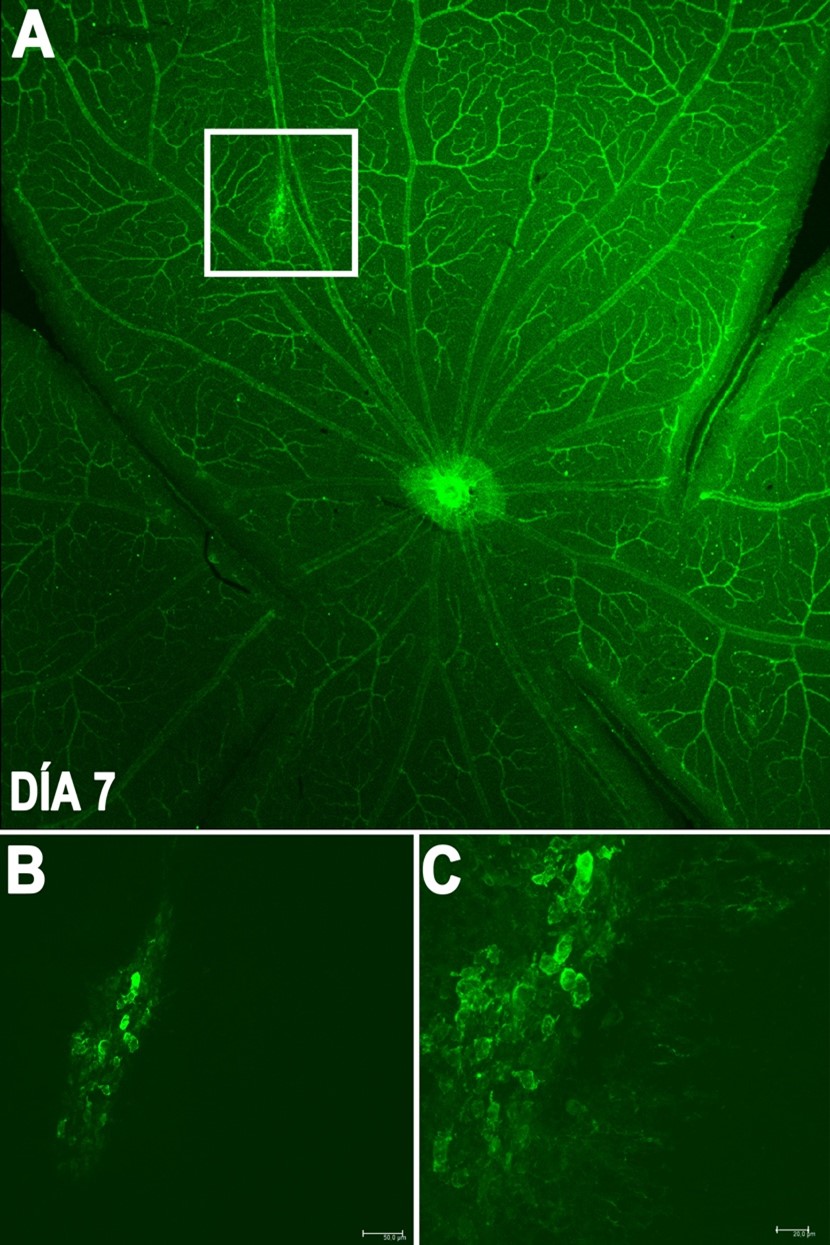
Generation of neovascularization by a laser system in the rat retina after 7 days.
Line 2:Ophthalmological therapy using interfering RNA (siRNA) and humanized monoclonal antibodies for retinal degenerative pathologies.
This line of research is based on the application of new molecules based on RNA interference and humanized monoclonal antibodies for chronic pathologies such as age-related macular degeneration (AMD), diabetic retinopathy and retinitis pigmentosa, which currently do not have satisfactory treatments
Line 3: DRUG4SIGHT: Light-regulated drugs to restore sight
In degenerative retinal diseases such as retinitis pigmentosa, photoreceptor cells are progressively lost, leading to visual impairment with limited treatment options. This line aims to characterize a series of light-sensitive active compounds that can photosensitize the retina, stimulating proteins still present in the degenerated retina and activating the remaining receptors of non-degenerated cells.
Line 4: New generation of multifunctional intraocular for prolonged and controlled release of therapeutic agents at the intraocular level (OCULUS)
To avoid or reduce effects associated with cataract surgery (pain, infection, inflammation and possible intraocular hemorrhage) it is necessary to administer anti-inflammatory and anti-infective drugs daily for at least 6-8 weeks after surgery. Topical administration of these drugs manages to retain less than 5% of the applied drug after passing through the cornea. For this reason, this line of research focuses on the development of new intraocular lenses that release drugs in a sustained manner in order to obtain a continuous therapeutic dose.
Line 5: Application of biofilms with Timolol for the control of intraocular pressure (OCULIPID)
Glaucoma is a progressive visual disease whose most important risk factor is elevated intraocular pressure. Current ocular pharmaceutical formulations have drawbacks in terms of their administration and therapeutic efficacy. This line of research focuses on the topical application of lipid biofilms formulated with prolonged release Timolol to control intraocular pressure in a preclinical experimental model of rabbit glaucoma
Line 6: Computational modeling environment for evaluating the safety and efficacy of peripheral nervous system stimulation
The objective of this line is to develop and make available to the scientific community a modular, integrated and multiscale computational modeling framework that will allow the design of safe and effective peripheral neurostimulators. The achievement of this line will be carried out through the three-dimensional reconstruction of the microstructure of stimulated nerves, through histological sections, by a computer-assisted anatomical dissection method. This is intended to improve the description of nerve fiber distribution, provide an accurate 3D representation of the sciatic nerves, and develop a computerized database of nerve fiber topography.
Line 7: Gene therapy with non-viral vectors for the treatment of neurodegenerative diseases
Nowadays,the cure to neurodegenerative diseases is a medical problem that is far from being solved. One approach to accomplish a solution is the capability to replace the defective genes involved in the development of such diseases for new fully functional genes capable of disrupting its progression. Gene therapy consists of the transfer of exogenous genetic material for the modification of cells with therapeutic or research purposes. In collaboration with the NanoBioCel group (Micro and Nano Technologies, Biomaterials and Cells Group) of the University of the Basque Country, our group is working on the development of new non-viral vectors to transfer genetic material inside the cells of the nervous system in order to find new therapies for neurological diseases. The great advantages of the non-viral vectors compared to other approaches are their very low or absent of immunogenic response in the receptor organisms, their biological and chemical safety for scientist and manufacturers, their capability to transport large genetic fragments inside the cells, and also, their ease of production, handling, storing and low cost of production.
Line 8: Development of Visual Neuroprosthesis
The NBIO multidisciplinary team led by Prof. Eduardo Fernandez Jover, is made up of a group of medical doctors, surgeons, biologists and engineers focused on the development and improvement of visual prostheses implanted in the visual cortex of blind patients or patients with low vision. The purpose of the group is to ensure that human patients achieve visual functionality that allows them to perform the usual tasks of daily life such as the ability to recognize objects, numerical and alphabetic symbols, as well as the ability to move independently through space. To achieve this aim, the team is involved in the optimization of the cortical implant protocol for surgery, the development and design of new electrodes that improve their biocompatibility with the nervous tissue, as well as in the study and analysis of the neural and perceptual responses of implanted human patients to stimulation through the neuroprosthesis. These studies are complemented by the development of artificial retinal models that attempt to reproduce the real activity of the ganglion cells of the retina in response to the presentation of different visual stimuli. Additionally, the group has built a smart study room that allows to evaluate the behavioral response of patients to the presentation of visual stimuli presented through the stimulation of the visual neuroprosthesis. The study room is a real-scale reproduction of a customizable outdoor space that allows human patients to move and navigate through it as if they would be walking through the city and guided by the presentation of stimuli through the implanted neuroprosthesis.
Line 9: Optogenetic approach in visual restoration
The emergence of optogenetics has revolutionized the field of neuroscience and has proved an invaluable tool for manipulating the activity in neurons through photostimulation with high temporal precision and genetic targeted specificity. Photosensitive channels such as channelrhodpsin-2 (ChR2) and the plethora of bioengineered variants which have emerged not only function as an explorative tool in neuroscience but have profound implications in therapy. We aim to use optogenetics as a viable and improved alternative to current approaches in visual restoration in patients with retinitis pigmentaria (RP) who have lost their photoreceptor layer. The introduction of optogenetic proteins into the residual retinal layers can prove clinically viable as a therapy capable of significant and meaningful changes to the quality of their vision and life. We express new improved optogenetic vatiants (CatCh and Chrimson) in the remaining intact retina essentially converting these cells into new basic photoresponsive tissue capable of reinitiating the visual signal and relaying it to the higher visual centers for processing.
N. Al Qtaish, I. Gallego, A.J. Paredes, I. Villate-Beitia, C. Soto-Sánchez, G. Martínez-Navarrete, M. Sainz-Ramos, T. B. López-Méndez, E. Fernández, G. Puras, J.L. Pedraz.
Nanodiamond Integration into Niosomes as an Emerging and Efficient Gene Therapy Nanoplatform for Central Nervous System Diseases.
ACS Appl. Mater. Interfaces 14(11):13665-13677 (2022).
E. Fernández, A. Alfaro, C. Soto-Sánchez, P. González-López, A.M. Lozano, S. Peña, M.D. Grima, A. Rodil, B. Gómez, X. Chen, P.R. Roelfsema, J.D. Rolston, T.S. Davis, R.A. Normann.
Visual percepts evoked with an intracortical 96-channel microelectrode array inserted in human occipital cortex.
J. Clin. Invest.131(23):e151331 (2021).
X. Chen, F. Wang, E. Fernández, P.R. Roelfsema.
Shape perception via a high-channel-count neuroprosthesis in monkey visual cortex.
Science 370(6521):1191-1196 (2020).
J. Sorinas, M.D. Grima, J.M. Ferrández, E. Fernández.
Identifying Suitable Brain Regions and Trial Size Segmentation for Positive/Negative Emotion Recognition.
Int. J. Neural Syst. 29(2):1850044 (2019).
I. Gallego, I. Villate-Beitia, G. Martínez-Navarrete, M. Menéndez, T. López-Méndez, C. Soto-Sánchez, J. Zárate, G. Puras, E. Fernández, J.L. Pedraz.
Non-viral vectors based on cationic niosomes and minicircle DNA technology enhance gene delivery efficiency for biomedical applications in retinal disorders.
Nanomedicine 17:308-318 (2019).
M. Mashal, N. Attia, G. Martínez-Navarrete, C. Soto-Sánchez, E. Fernández, Grijalvo S, R. Eritja, G. Puras, J.L. Pedraz.
Gene delivery to the rat retina by non-viral vectors based on chloroquine-containing cationic niosomes.
J. Control Release 304:181-190 (2019).
A. Lozano, C. Soto-Sánchez, J. Garrigós, J.J Martínez, J.M: Ferrández, E. Fernández.
A 3D Convolutional Neural Network to Model Retinal Ganglion Cell’s Responses to Light Patterns in Mice.
Int. J. Neural Syst. 28(10):1850043 (2018).
M. Mashal, N. Attia, G. Puras, G. Martínez-Navarrete, E. Fernández, J.L. Pedraz.
Retinal gene delivery enhancement by lycopene incorporation into cationic niosomes based on DOTMA and polysorbate 60.
J. Control Release 254:55-64 (2017).
M. Izquierdo-Serra, A. Bautista-Barrufet, A. Trapero, A. Garrido-Charles, A. Díaz-Tahoces, N. Camarero, S. Pittolo, S. Valbuena, A. Pérez-Jiménez, M. Gay, A. García-Moll, C. Rodríguez-Escrich, J. Lerma, P. de la Villa, E. Fernández, M.Á. Pericàs, A. Llebaria, P. Gorostiza.
Optical control of endogenous receptors and cellular excitability using targeted covalent photoswitches.
Nat. Commun. 7:12221 (2016).
J.M. Ayuso, M. Virumbrales-Muñoz, A. Lacueva, P.M. Lanuza, E. Checa-Chavarria, P. Botella, E. Fernández, M. Doblare, J.S. Allison, R.M. Phillips, J. Pardo, L.J. Fernández, I. Ochoa.
Development and characterization of a microfluidic model of the tumour microenvironment.
Sci. Rep. 6:36086 (2016).
Neural Active Visual Prosthetics for Restoring Function (H2020). Funding agency: European Commission. Duration 2022 – 2024
PI: Eduardo Fernández Jover
Light-regulated drugs to restore sight (HR18-00501). Funding agency: Foundation La Caixa, Spain. Duration 2020 – 2022
PI: Eduardo Fernández Jover
European Network for integrated Training of innovative therapies for Vision Restoration (H2020). Funding agency: European Commission. Duration 2020 – 2024
PI: Eduardo Fernández Jover
Computational modeling framework for safety and efficacy assessment of PNS Stimulation (CNRS US-Spain research proposal). Funding agency: NSF (USA) and Ministry of Health, Spain. Duration 2018 – 2022
PI: Eduardo Fernández Jover
Innovative Neurotechnology for Society. Funding agency: Netherlands Organisation for Scientific Research. Duration 2020 – 2027
PI: Eduardo Fernández Jover
